VHH antibodies against new coronavirus, SARS-CoV-2
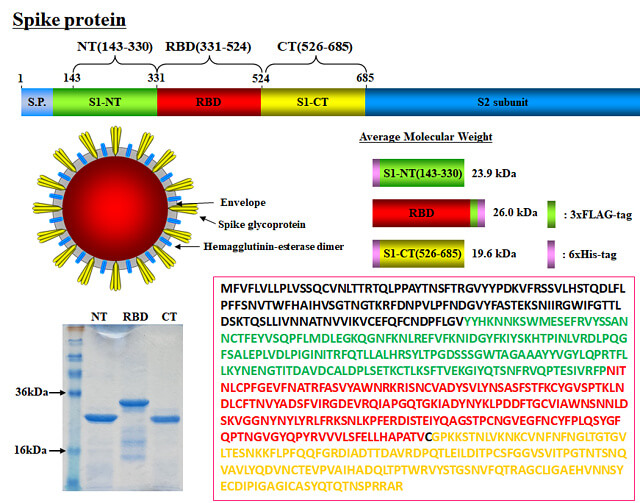
Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1 complete genome provided by the National Center for Biotechnology Information (NC_045512.2) was analyzed and the gene of the receptor binding domain (RBD) of spike protein was synthesized.
RBD binds to ACE2 (angiotensin converting enzyme 2), which is a receptor for SARS-CoV-2 that is expressed on the human cell surface. The RBD gene was ligated to pET22b E. coli protein expression vector and expressed as 6xHis-tag fusion protein.
Affinity purification using Ni-NTA was performed and used as a target antigen for VHH antibody screening. As a control, 6xHis fusion proteins on the N-terminal region and the C-terminal region of the S1 subunit were also prepared.
Based on a VHH antibody-displaying phage library having a diversity of 20 billion, VHH-displaying phages having binding properties were concentrated by a three-round panning method, and then cloned.
The FR3-CDR3-FR4 gene of the obtained VHH antibody clone was amplified by PCR, ligated with the FR1-CDR1-FR2-CDR2 gene having diversity of 1 billion, inserted into a phage display vector, and transformed into E. coli. As the result, we created a SARS-CoV-2 specific CDR3 preservation library with about 100 million diversity.
From the SARS-CoV-2-specific CDR3- preserved library, VHH-displaying phages that had binding properties were concentrated by two rounds of panning and then cloned. As a result, clones having 19 different amino acid sequences were obtained.
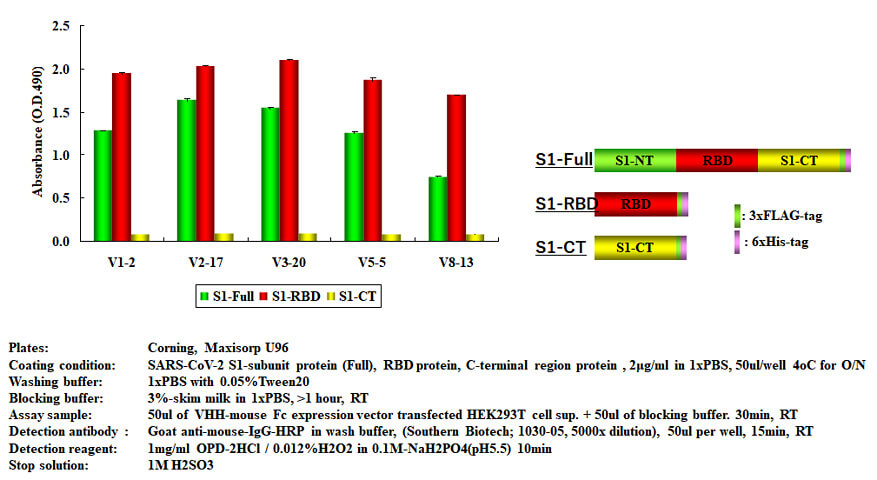
Five clones were selected from these, VHH-Fc (Fc is derived from mouse IgG1) expression constructs were prepared, and HEK293T cells were transiently transduced, and ELISA was performed using the culture supernatant.
As a result of examining the binding to the full length (S1-Full), RBD, and C-terminal side, it was confirmed that the all VHH clones specifically bind to RBD and S1-Full having RBD.
It was also confirmed that those also bind to the S1 subunit protein and RBD that were transiently expressed in HEK293T cells.
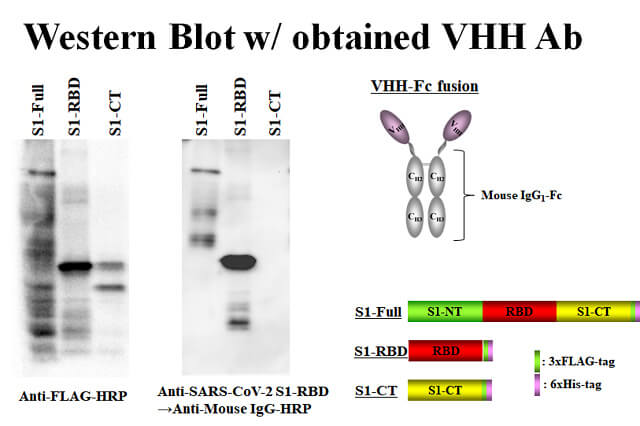
As a result of verifying whether the obtained VHH clone can be used in Western blotting, it was confirmed that it could be used.
(the display data used 2-mercaptoethanol-treated antigen, but it can be detected without it)