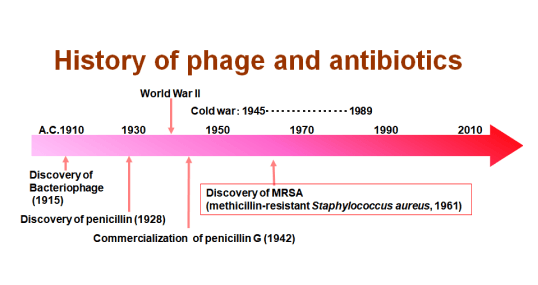
Phage has been studied extensively mainly in the former Soviet Union and Eastern Europe, but since it is effective only for specific bacteria, research on phage has decreased due to the emergence of antibiotics that can respond to many infectious diseases, especially in the West.
The emergence of this antibiotic has overcome the terrible infectious diseases that have killed many humans, such as sepsis and tetanus, and have dramatically advanced modern medicine.
Antibiotics, called "miracle drugs," were effective against a variety of infectious diseases, but were eventually used in large quantities, resulting in resistant bacteria that could not be killed with antibiotics, and now, how to deal with drug-resistant bacteria is becoming a big problem.
Phage therapy using phage has been practiced in eastern countries such as Russia, Georgia (now Georgia), and Poland for a long time, and is currently being pharmaceuticalized in those countries.
Phage R & D has been activated in the United States and Europe in the latter half of the 2010s, following a case in the United States where phage treatment showed dramatic therapeutic effects.
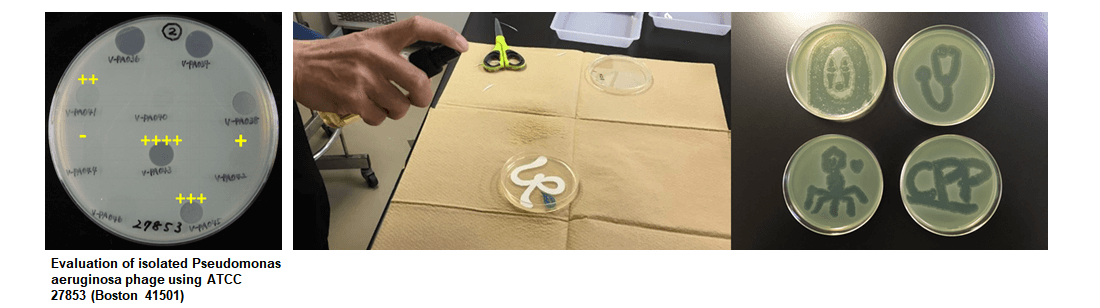
A phage and antibiotics were simultaneously injected into a man infected with a Pseudomonas aeruginosa bacterium that is highly resistant to antibiotics from an artificial blood vessel used in heart surgery.
As a result, a few weeks later the infection was cured.
However, the number of clinical studies is limited, and phage therapy is in the research stage and has not yet reached practical use.
In addition to medical use, practical use has begun in fields such as food safety.
Application to Listeria monocytogenes.
When a person becomes infected with Listeria monocytogenes, healthy adults often end up with mild gastrointestinal symptoms or asymptomatic. However, the elderly, immunocompromised, infants, etc. may suffer from severe symptoms such as meningitis and sepsis, and the fatality rate is said to be 20-30%.This phage preparation against Listeria monocytogenes has already been formulated in the United States as a safe product approved by the US Food and Drug Administration (FDA).
This phage product has emerged because it is easier to be approved as a food ingredient or additive than it is to be a pharmaceutical, and the need for new innovations that reduce the risk of food contamination.
However, phage products have only recently appeared on the market.
Thus, in the latest global trend, phage preparations will be used not only as human medicines but also as quasi-drug disinfectants and veterinary medicines. If progress is made, it will play a part in the antibacterial agent market, and development competition has begun in each country.